
Biofluid Mechanics
Main Topics
Motion of the vitreous body induced by eye
rotations
- Introduction to the problem
-
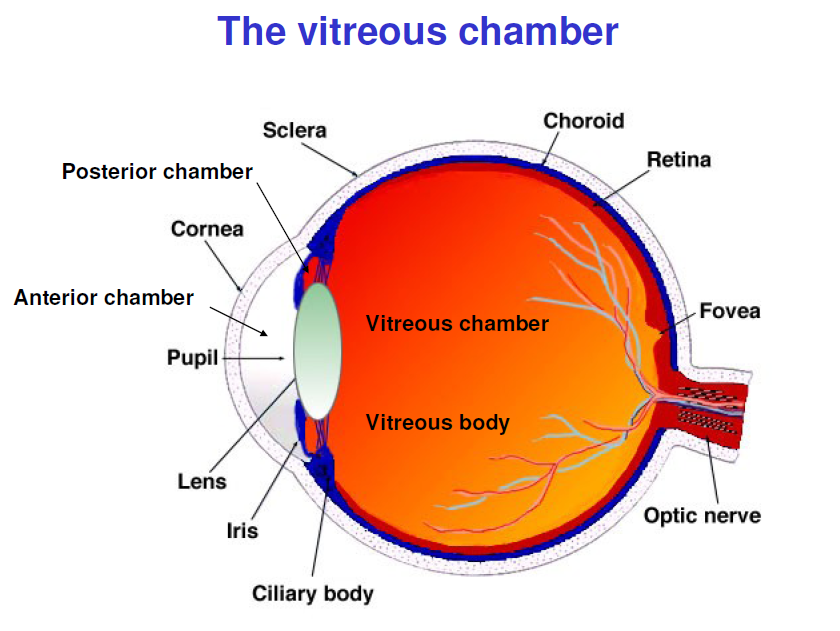 Eye biomechanics is a rapidly growing area in biomechanics. Indeed, both eye physiology and the generation of various eye pathological states are inherently associated with mechanics of solids, fluids and materials. Such a growing interest in the field is corroborated by the increasing number of specific scientific meetings on this topic recently organised, primarily in the United States, and by the fact that eye biomechanics has started appearing in introductory textbooks of biomechanics
(Ethier & Simmons 2007). A relatively recent comprehensive review on eye biomechanics can be found in Ethier et al. (2004).
A very large body of biomechanical literature has been produced aimed at understanding the mechanisms controlling the intraocular pressure (IOP) and the related issue of pathogenesis of glaucoma, i.e. cell death in the optic nerve (e.g. Stamer et al. 2008; Sigal et al. 2007; Band et al. 2009 and references therein). The thermally
driven flow of the aqueous humour in the anterior chamber of the eye has also been thoroughly studied both theoretically and numerically (e.g. Fitt & Gonzalez 2006; Heys & Barocas 2002). Another purely mechanical problem related to the eye is tonometry, i.e. the way in which the IOP is clinically measured (e.g. Gonzalez & Fitt 2003).
Eye biomechanics is a rapidly growing area in biomechanics. Indeed, both eye physiology and the generation of various eye pathological states are inherently associated with mechanics of solids, fluids and materials. Such a growing interest in the field is corroborated by the increasing number of specific scientific meetings on this topic recently organised, primarily in the United States, and by the fact that eye biomechanics has started appearing in introductory textbooks of biomechanics
(Ethier & Simmons 2007). A relatively recent comprehensive review on eye biomechanics can be found in Ethier et al. (2004).
A very large body of biomechanical literature has been produced aimed at understanding the mechanisms controlling the intraocular pressure (IOP) and the related issue of pathogenesis of glaucoma, i.e. cell death in the optic nerve (e.g. Stamer et al. 2008; Sigal et al. 2007; Band et al. 2009 and references therein). The thermally
driven flow of the aqueous humour in the anterior chamber of the eye has also been thoroughly studied both theoretically and numerically (e.g. Fitt & Gonzalez 2006; Heys & Barocas 2002). Another purely mechanical problem related to the eye is tonometry, i.e. the way in which the IOP is clinically measured (e.g. Gonzalez & Fitt 2003).
The vitreous humour is an avascular material of gel-like consistence and viscoelastic properties. It contains 99% water, 0.9% low molecular weight solutes, 0.1%
macromolecules, such as collagen and hyaluronic acid and other non-collagenous soluble proteins (Sebag 1989). The concentration of collagen fibrils, which is mainly responsible for the viscoelasticity of the vitreous, is higher in the peripheral region, in contact with the retina, which is denoted as vitreous cortex. Lee et al., 1992 first performed a systematic study of the rheological properties of the vitreous humour. They employed a magnetic microrheometer specifically designed for the
purpose, which allowed working with very small samples of the vitreous. The vitreous was characterised according to a 4-parameter Burgers model, consisting of a Maxwell element in series with a Kelvin element. The vitreous was divided into three different areas (anterior, central and posterior), in which vitreous properties were separately measured. More recently Nickerson et al. (2008) performed rheological measurements of bovine and porcine vitreous. The authors point out that
vitreous properties vary significantly in time after removal from the eye, stressing the difficulty of obtaining reliable measures ex vivo. They provide elastic values of the vitreous behaviour which are significantly higher than previously reported. A large body of literature exists on fluids to be used for artificial vitreous replacement
after vitrectomy. A review on the topic can be found in Soman & Banerjee (2003). Recently Kummer et al. (2007) presented a protocol to create an artificial vitreous phantom to be employed for in laboratory experiments.
The importance of understanding vitreous dynamics as a response to eye movements has been recognised for long time, especially in connection with the generation of RRD. Back in 1933 Lindner performed a series of experiments, employing a cylindrical container filled with water, whose inner wall was linen by a thin transparent membrane, simulating the retina. In the membrane a small hole was produced mimicking a retinal tear. The container was subjected to controlled movements, either translations or rotations, and the author observed both fluid motion and the possible tendency of the flow to produce detachment of the membrane from the wall. The very preliminary conclusion obtained by inspection of the system behaviour was that only rotations produce fluid motion relative to the container wall, which might induce a peeling of the membrane. Rosengren and Östrelin (1976), who performed similar experiments, stress the importance of vitreous motion close to the wall to produce a RRD. They also first speculate that accounting for the real geometry of the vitreous chamber, and in particular the presence of the lens
in the anterior part, might lead to significantly different results. More recently, David et al. (1998) have reconsidered the problem of vitreous dynamics induced by eye movements, within the context of a bioengineering approach.
They used an analytical model, describing the vitreous as a viscoelastic material with the characteristics measured by Lee et al. (1992). In their model the vitreous chamber is described as a sphere performing small amplitude harmonic torsional oscillations about an axis passing through the sphere centre. The authors describe in detail the velocity profiles generated in the sphere. Their main conclusion is that the maximum wall shear stress (WSS) increases with the sphere radius, and this
finding is provided as a possible explanation for the fact that in myopic eyes (which are typically larger than normal eyes) RD is more frequently developed.
Finally, Walton et al. (2002) performed ultrasound scan recordings of vitreous motion induced by eye rotations, with the purpose of testing the hypothesis that the biomechanical properties of the vitreous change with age. The authors considered eyes with and without PVD. They tracked the motion of speckles in the vitreous,
focussing on the angle travelled by the speckle after a single saccadic rotation and the corresponding duration of motion, as a function of the radial position of the speckle within the vitreous. Their results confirm the role of ageing in determining vitreous mechanical properties. Very limited work has been carried out so far on the biomechanical aspects of RRD. It is known that pre-requisites for the development of RRD are i) liquefaction of the vitreous and ii) tractional forces that produce a retinal break. However, the majority of retinal breaks do not result in RRD. In the general population, RRD occurs in about 12 out of 100,000 people (0.01% annual risk) with a lifetime risk (up to 60 years of age) of 0.6%, and is one of the most frequent causes of blindness in Western countries (Sodhi et al., 2008). A few experimental data on the adhesive force between the retina and the pigmented epithelium are available (see for instance Kita et al, 1992 and Yao et al. 1994).
Traction on the retina during eye rotations has been studied by various BioMechEye participants (Repetto et al., 2002; Colangeli et al., 2007). Their works are briefly described in section 9 of the proposal.
No models exist of the tractions generated on the retina due to vitreous syneresis. From the microscopic point of view this process consists of the aggregation of densely packed collagen fibrils into bundles of parallel macroscopic fibers (Sebag, 1989). Collagen is the main constituent of most living tissues. It has recently attracted great attention (e.g. Fratzl, 2008) because of its mechanical properties, which are relevant to any bio-mechanical model. As a living material it evolves with
time as it happens in the vitreous gel, where alterations in the normal hyaluronic acid-collagen association result in the processes of synchysis and syneresis. Synereris could be modelled by treating the vitreous as an ideal homogeneous material, characterized by a structural matrix made of collagen fibrils which shrinks while releasing a fluid, and employing a material remodelling theory (Di Carlo & Quiligotti, 2002; Di Carlo, 2005). To our knowledge, the only theoretical approach to the mechanisms leading to RDD once a retinal break has formed is due to González (2004), who proposed a mechanism based on the role of surface tension effects. Presently A. Fitt from the University of Southampton is supervising a PhD thesis concerned with the
mathematical modelling of RRD, accounting for the effect of liquefied vitreous motion over a retinal tear.
- Experimental models: simplified spherical domain
- Experimental models: realistic shape
- Drug delivery within the vitreous chamber
Abnominal Aortic Aneurisms (AAA): a fluid mechanics perspective
- Impact of the AAA on the Human Health

 Eye biomechanics is a rapidly growing area in biomechanics. Indeed, both eye physiology and the generation of various eye pathological states are inherently associated with mechanics of solids, fluids and materials. Such a growing interest in the field is corroborated by the increasing number of specific scientific meetings on this topic recently organised, primarily in the United States, and by the fact that eye biomechanics has started appearing in introductory textbooks of biomechanics
(Ethier & Simmons 2007). A relatively recent comprehensive review on eye biomechanics can be found in Ethier et al. (2004).
A very large body of biomechanical literature has been produced aimed at understanding the mechanisms controlling the intraocular pressure (IOP) and the related issue of pathogenesis of glaucoma, i.e. cell death in the optic nerve (e.g. Stamer et al. 2008; Sigal et al. 2007; Band et al. 2009 and references therein). The thermally
driven flow of the aqueous humour in the anterior chamber of the eye has also been thoroughly studied both theoretically and numerically (e.g. Fitt & Gonzalez 2006; Heys & Barocas 2002). Another purely mechanical problem related to the eye is tonometry, i.e. the way in which the IOP is clinically measured (e.g. Gonzalez & Fitt 2003).
Eye biomechanics is a rapidly growing area in biomechanics. Indeed, both eye physiology and the generation of various eye pathological states are inherently associated with mechanics of solids, fluids and materials. Such a growing interest in the field is corroborated by the increasing number of specific scientific meetings on this topic recently organised, primarily in the United States, and by the fact that eye biomechanics has started appearing in introductory textbooks of biomechanics
(Ethier & Simmons 2007). A relatively recent comprehensive review on eye biomechanics can be found in Ethier et al. (2004).
A very large body of biomechanical literature has been produced aimed at understanding the mechanisms controlling the intraocular pressure (IOP) and the related issue of pathogenesis of glaucoma, i.e. cell death in the optic nerve (e.g. Stamer et al. 2008; Sigal et al. 2007; Band et al. 2009 and references therein). The thermally
driven flow of the aqueous humour in the anterior chamber of the eye has also been thoroughly studied both theoretically and numerically (e.g. Fitt & Gonzalez 2006; Heys & Barocas 2002). Another purely mechanical problem related to the eye is tonometry, i.e. the way in which the IOP is clinically measured (e.g. Gonzalez & Fitt 2003).